New RNA Discovery Explains Why Oral Cancer Resists Chemotherapy
By hijacking a key growth receptor under low-oxygen conditions, this newly discovered RNA molecule helps oral cancer cells survive chemotherapy
CHINA, September 30, 2025 /EINPresswire.com/ -- Hypoxia, or low oxygen in the body, is common in solid tumors. Now, researchers have identified that an RNA molecule, EUDAL, enables oral cancer cells to resist chemotherapy by keeping a growth receptor permanently switched on under low-oxygen conditions. This drives autophagy, a cellular process that helps tumors evade treatment. These findings suggest that EUDAL is both a warning sign for poor treatment response and a potential therapeutic target, offering new hope against stubborn cancers.Oral cancer is one of the most common head and neck cancers worldwide, with hundreds of thousands of new cases diagnosed every year. Despite advances in surgery, radiotherapy, and chemotherapy, survival rates remain poor. One of the main challenges is that tumors quickly adapt and develop resistance to drugs that previously controlled them.
A key factor behind this resistance is hypoxia—or the shortage of oxygen that develops inside tumors as they grow. Hypoxia not only promotes aggressive cancer behavior but also makes treatments less effective. Scientists have long suspected that hypoxia interacts with critical growth pathways in cancer cells, but the exact mechanisms have remained unclear.
Now, a new study published online on 12 September 2025, in volume 17, issue 1, in the International Journal of Oral Science, addresses this and uncovers an unexpected answer. The study was led by Distinguished Professor Zhiyuan Zhang and Associate Professor Qin Xu at the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, China, and reports that hypoxia can directly activate the epidermal growth factor receptor (EGFR)—a protein that normally drives cell growth and survival when switched on by external signals. Here, however, EGFR becomes active without its usual triggers.
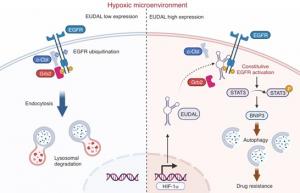
In a remarkable discovery, the team identified a previously unknown long noncoding RNA (lncRNA), which they named EUDAL—short for EGFR ubiquitination- and degradation-associated lncRNA. Unlike messenger RNAs that code for proteins, lncRNAs regulate other molecules inside cells. Normally, EGFR is kept under control by a cellular “tagging” system, where helper proteins such as c-Cbl/Grb2 attach small molecular labels that signal it for disposal. EUDAL blocks this step by binding to EGFR and preventing its breakdown. As a result, EGFR remains permanently active, triggering downstream signaling pathways (STAT3/BNIP3; Signal Transducer and Activator of Transcription 3) and promoting autophagy—a recycling mechanism that cancer cells hijack to survive chemotherapy.
“We were surprised to discover that oxygen shortage alone was enough to switch on EGFR in oral cancer cells,” says Prof. Zhang. “This noncanonical activation gives tumors a survival advantage and helps explain why many patients do not respond to chemotherapy.”
To test the role of EUDAL, the researchers carried out experiments in both, cell and animal models. They found that oral cancer cells with high EUDAL levels resisted cisplatin, a standard chemotherapy drug. However, when EUDAL was blocked, cancer cells regained their sensitivity to the drug treatment.
Animal studies demonstrated similar results. Tumors rich in EUDAL continued to grow even after cisplatin therapy, but when combined with inhibitors of STAT3 or autophagy, chemotherapy became effective again, significantly reducing tumor size.
The team also examined tumor samples from patients undergoing platinum-based chemotherapy. Those with higher levels of EUDAL, active EGFR, and STAT3 were far more likely to have poor responses, while patients with lower levels responded better to treatment.
Discussing these results further, Dr. Xu says, “Our results suggest that EUDAL is not just a marker but a driver of resistance. In practice, measuring EUDAL levels could help predict which patients are unlikely to benefit from standard chemotherapy, allowing doctors to choose alternative or combination strategies.”
These findings also reshape our understanding of cancer biology. EGFR is a well-known cancer driver and a common target for therapies, but its activation was previously thought to depend on mutations or external growth factors. This study shows that the tumor microenvironment—in this case, lack of oxygen—can also fuel EGFR activity through a novel RNA-based mechanism. However, further research is needed before these insights can be translated into clinical practice.
Overall, the findings of this study highlight EUDAL as both a biomarker of poor treatment response and a potential therapeutic target. Drugs that block EUDAL or its downstream signaling could one day be paired with chemotherapy to outsmart resistant tumors. By revealing how tumors exploit EUDAL to survive, this study exposes a hidden vulnerability in oral cancer. Cutting off this escape route may offer patients a better chance at successful treatment and longer survival.
***
Reference
Title of original paper: LncRNA EUDAL shapes tumor cell response to hypoxia induced constitutive EGFR activation and promotes chemoresistance in oral cancer
Journal: International Journal of Oral Science
DOI: 10.1038/s41368-025-00396-2
About Shanghai Jiao Tong University, School of Medicine, Shanghai, China
Shanghai Jiao Tong University School of Medicine (SJTUSM), located in Shanghai, China, is a premier institution dedicated to medical education, research, and clinical care. Established in 1952, SJTUSM offers a wide range of programs in medicine, dentistry, public health, and related biomedical fields. The school is renowned for its strong emphasis on innovative research, advanced clinical training, and international collaboration. With multiple affiliated hospitals and research centers, SJTUSM plays a vital role in advancing healthcare in China and nurturing highly skilled medical professionals.
Website: https://www.shsmu.edu.cn/english/
About Prof. Zhiyuan Zhang from Shanghai Jiao Tong University, School of Medicine, Shanghai, China
Zhiyuan Zhang, MD, Ph.D., is a Distinguished Professor at Shanghai Jiao Tong University School of Medicine and an Academician of the Chinese Academy of Engineering. He is Chief Surgeon and doctoral supervisor in Oral & Maxillofacial Surgery and previously served as Dean of the Ninth People’s Hospital (1998–2014). He leads national centers and labs, including the National Clinical Medical Research Center for Dental Disease. With over 334 publications (124 SCI-indexed) and 19 major grants, his research covers clinical and basic studies of oral/head–neck tumors, hemangiomas, and vascular malformations, including China’s first prospective trial of neoadjuvant chemotherapy in oral cancer.
About Dr. Qin Xu from Shanghai Jiao Tong University, School of Medicine, Shanghai, China
Qin Xu, Ph.D., is Associate Professor in the Department of Bioinformatics and Biological Statistics, Shanghai Jiao Tong University, China. He has published 62 scientific papers, accumulating over 1,200 citations. His work spans computational structural biology, including molecular dynamics simulations, protein structure-function relationships, and protein-drug binding studies. With more than a decade of research experience since at least 2005, he brings strong expertise in modeling how biological molecules behave and interact—a skillset that helps bridge lab findings with clinical insights.
Funding information
This work was supported by the National Natural Science Foundation of China (82273095, 82203614); the Shanghai Sailing Program (22YF1421600).
Yini Bao
Bone Research Editorial Office
2885546461 ext.
br@scu.edu.cn
Visit us on social media:
X
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.